Cloneable Nanoparticles
Published on January 8, 2020 6:13 pm MT Updated on February 16, 2024 3:25 pm MT
PROJECT SUMMARY: The objective of this proposal is to address the contrast problem in images formed by X-Ray, electron, or other scattering based illumination modalities. Briefly, images are made by contrast. In other words, we only see (or acquire information) on things that are distinguishable from their background. In all forms of biological imaging, many things are visible, yet many other things remain camouflaged or indistinguishable from the background. For instance, in an X-ray, it’s easy to see bones, but not so easy to see muscles, fat or skin. This is also true in microscopic images, where it’s often easy to see the edges of cells, but much harder to see the details inside cells. Green Fluorescent Protein (GFP) and related fluorescent proteins complement small molecule stains and dyes to essentially solve the contrast problem in optical imaging. For imaging based on X-rays or electrons, however, there are no clonable contrast agents. Clonable contrast (a GFP homolog) in X-Ray or electron-based imaging could be understood as a `clonable nanoparticle.’ Such a nanoparticle would scatter incident radiation and would be made by a protein that can be genetically fused to other proteins of interest. Three discrete chemical activities are needed for such a clonable nanoparticle: (1) conversion of bioavailable inorganic ions to insoluble nanoparticulate form; (2) maintenance of the nanoparticle at the protein that synthesizes it; (3) size control of the nanoparticle, where 5nm diameter is suggested as an ideal size.
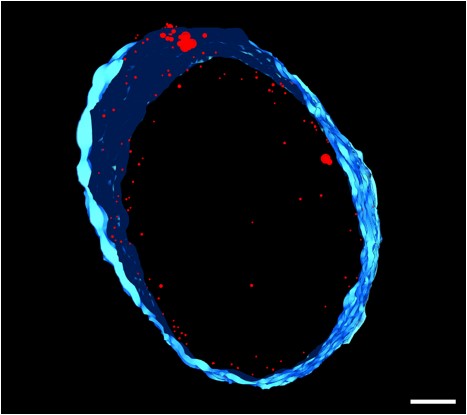
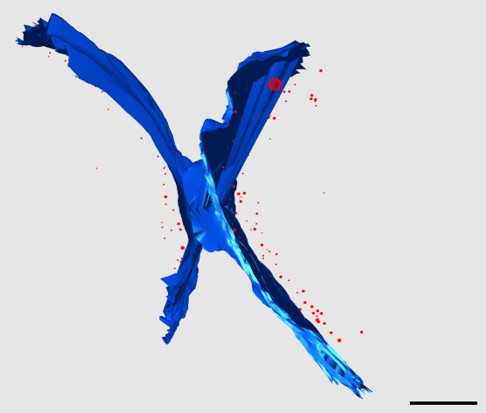
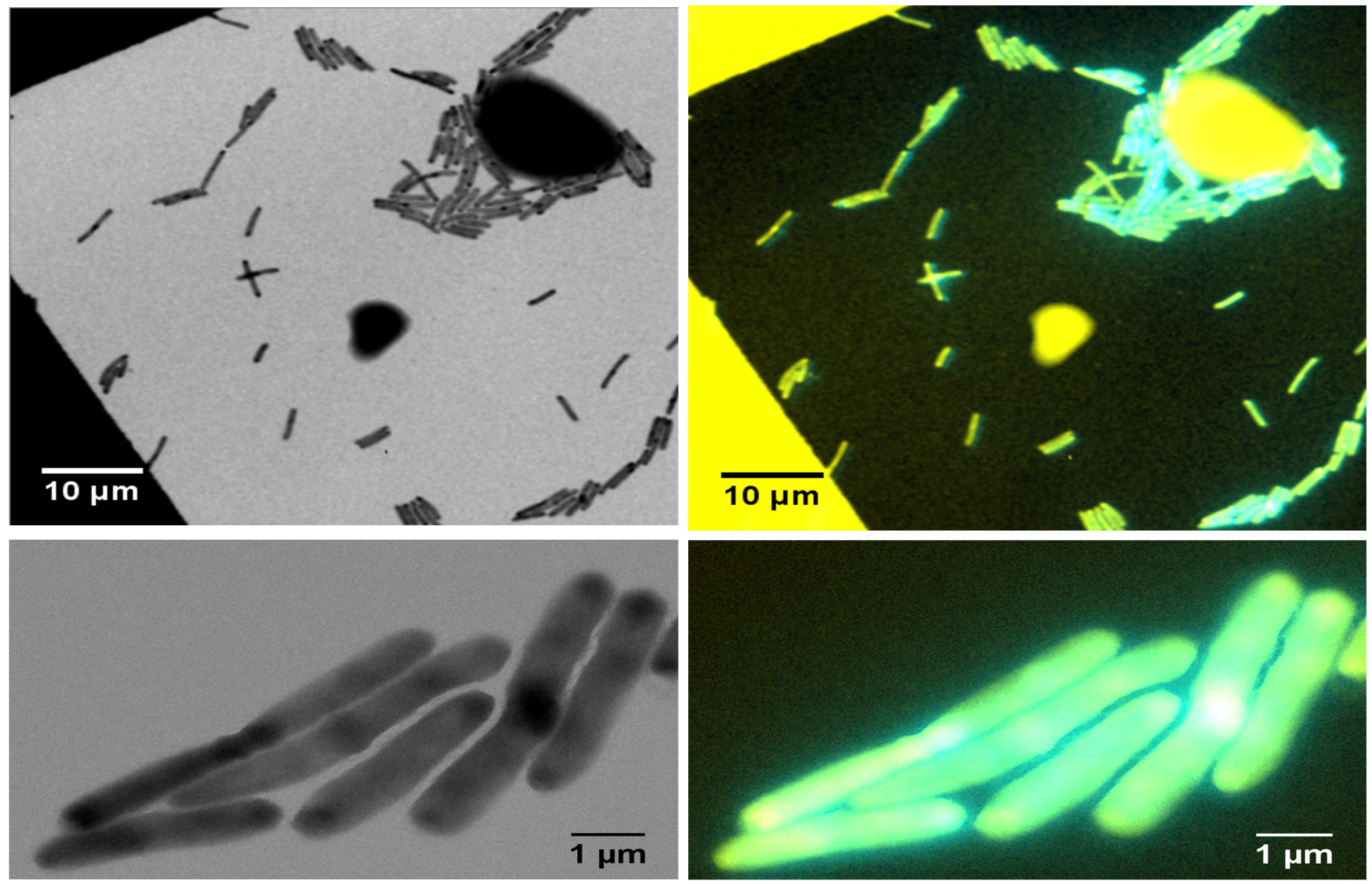
We recently isolated a metalloid reductase that appears to partially fulfill each of these chemical requirements. We propose to build on this finding to create a pipeline that will produce many clonable nanoparticles of distinct size, shape or elemental composition. The most broadly useful clonable nanoparticles will simultaneously incorporate X-ray/electron scattering, magnetism and fluorescence. Such nanoparticles could serve as `universal clonable contrast agents’ functioning in optical, MRI, X-Ray and electron imaging. Such a tool would greatly facilitate integrative and/or correlative multiscale bioimaging, integrating information from multiple imaging modalities. The proposed work will proceed in three specific aims. Candidate clonable nanoparticles will be identified and refined in Aims 1a and 1b. Refinement of our existing clonable nanoparticle candidate will proceed through directed evolution methods of saturation mutagenesis, DNA shuffling, and random mutagenesis. Isolation of additional variants and novel enzymes will begin with field samples collected in areas with persistent environmental metal contamination. Candidate clonable nanoparticles will be assessed in in vitro, in situ and in vivo within Aim 2 using 3 model and experimental systems we have identified to assess portability across species and suitability in in vivo, in situ, and in vitro chemical environments.